Description
Product References
Expiration Date : 02/2026
Genabio COVID-19 Rapid Self-Test is easy to use and give you accurate rapid results in as few as 15 minutes in the comfort of your own home. This test is for over-the-counter (OTC) at home and other non-laboratory sites for patients 2 years or older who are suspected of COVID-19 by a healthcare provider within the first 7 days of symptom onset. This test is also for nonprescription or over-the-counter (OTC) at home and other non-laboratory sites for screening of individuals 2 years or older without symptoms or other reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours and no more than 48 hours between tests. No prescription is required and Genabio is available to purchase over-the-counter (OTC).
Kit contains:
- COVID-19 Test Cards
- Swab
- Pre Filled Tube
- Instructions for Use
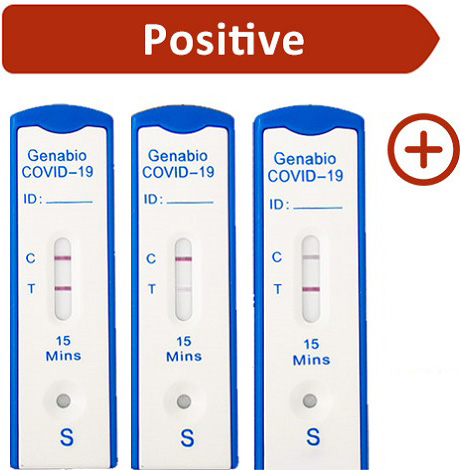
Control (C) line and Test (T) line both appear as pink-colored lines in the show window.
Note: Any faint visible pink color Test (T) line should be interpreted as positive, when the Control (C) line is also present. The Test (T) line may vary in shade an intensity (light or dark, weak or strong) depending on the concentration of antigen present in the sample. The intensity of the Control (C) line should not be compared to that of the Test (T) line for interpretation of the test result.
You do not need to perform repeat testing if you have a positive result at any time.
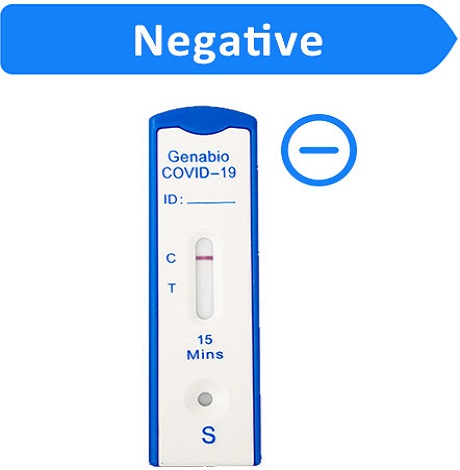
If the Control (C) line is visible, but the Test (T) line is not visible, the test is negative.
To increase the chance that the negative result for COVID-19 is accurate, you should:
•Test again in 48 hours if you have symptoms on the first day of testing.
•Test 2 more times at least 48 hours apart if you do not have symptoms on the first day of testing.
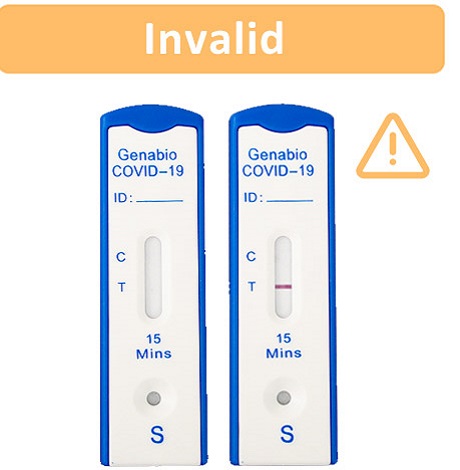
If no line appears in the Control (C) area, the test results are invalid regardless of the presence or absence of a line in the Test (T) area. An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, re-test with a new swab and new test device.
This Product has not been FDA-cleared or approved but has been authorized by FDA under an Emergency Use Authorization (EUA). This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
Images shown may vary slightly from items shipped. Information supplied is deemed to be accurate but cannot be guaranteed.















